NASA scientists reported that the Ozone Hole over Antarctica in September 2013 is slightly smaller and has slightly more ozone than recent year measurements. This is good news. Here is why.
 Oxygen is the third most abundant element in the universe behind Hydrogen and Helium. It is the second most abundant element by volume in our atmosphere making up 20.8% of the air we breathe. It is normally found in a two molecule state in the air as that is colorless, odorless, and tasteless and the formula O2. Because it it highly reactive with other elements, it is also found in many other compounds. By mass, it makes up 49.2% of mass of Earth’s crust and 88.8% of the oceans.
Oxygen is the third most abundant element in the universe behind Hydrogen and Helium. It is the second most abundant element by volume in our atmosphere making up 20.8% of the air we breathe. It is normally found in a two molecule state in the air as that is colorless, odorless, and tasteless and the formula O2. Because it it highly reactive with other elements, it is also found in many other compounds. By mass, it makes up 49.2% of mass of Earth’s crust and 88.8% of the oceans.
 Ozone is a form of Oxygen with three molecules instead of two with the formula O3. It is a pale blue gas and has a pungent smell. You might recall the smell if you have experienced electrical discharges and sparks. It is a much more active element chemically than the two molecule form of Oxygen. Near the Earth’s surface, Ozone damages mucous and respiratory tissue. It damages plants and causes damage to rubber. It is a biological hazard and strong pollutant at ground levels. It occurs in much smaller concentrations than the two molecule version. But, since it is highly reactive, small concentrations have a large impact.
Ozone is a form of Oxygen with three molecules instead of two with the formula O3. It is a pale blue gas and has a pungent smell. You might recall the smell if you have experienced electrical discharges and sparks. It is a much more active element chemically than the two molecule form of Oxygen. Near the Earth’s surface, Ozone damages mucous and respiratory tissue. It damages plants and causes damage to rubber. It is a biological hazard and strong pollutant at ground levels. It occurs in much smaller concentrations than the two molecule version. But, since it is highly reactive, small concentrations have a large impact.
High in the stratosphere, Ozone is a very beneficial and important substance. It interacts with ultraviolet-B radiation from the Sun and prevents it from reaching the surface. This is a protection to plant and animal life. So, Ozone is both helpful and harmful to living things depending on where it is found.
Scientists regularly measure the amount of Ozone in the stratosphere in order to know the concentrations around the Earth. They produce maps of the concentrations like this one from September of 2013. The blue and violet regions are where Ozone is less abundant. This map shows a large Ozone Hole in the atmosphere over the Antarctic and reaching into S. America. The areas on the ground in that hole are less protected from ultraviolet-B rays. This is not a good thing.
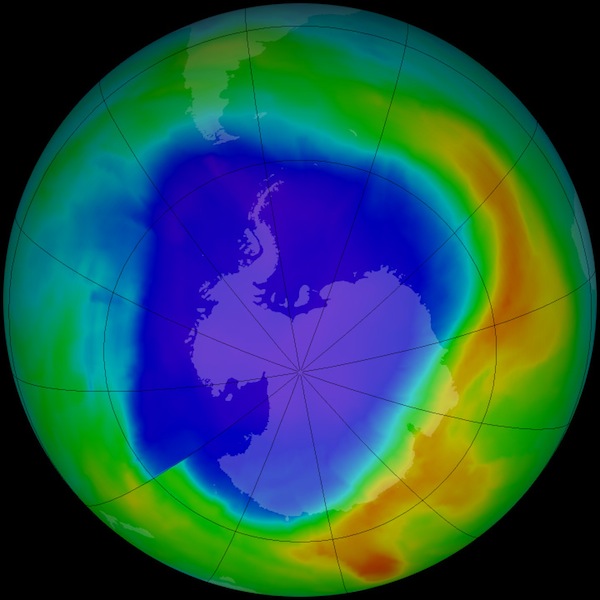
Dobson Units of Ozone Concentration
Monitoring of the ozone is carried out by the Ozone Monitoring Instrument (OMI) on NASA’s Aura satellite and the Ozone Monitoring and Profiler Suite (OMPS) on the NASA-NOAA Suomi NPP satellite. Measurements from these satellites allow the scientists to calculate the area of the hole marked in blue above. And, the amount of ozone in the atmosphere is calculated in Dobson Units DU. What is a Dobson Unit of ozone? Without getting into the technical details, let me give you an example.
 If you could get all of the ozone gas molecules to drop to the surface of the Earth at 0˚C and 1 atmosphere of air pressure and form a layer, it would be an average thickness of only 3 millimeters. That is two pennies deep. Scientists say Earth’s normal ozone concentration is 300 DU for that thickness. That is represented by the large green areas on the global image above. The Ozone Hole in blue and violet has less ozone concentration in the atmosphere. The value is down to about 100 DU. That is a third of what is found over the rest of the globe.
If you could get all of the ozone gas molecules to drop to the surface of the Earth at 0˚C and 1 atmosphere of air pressure and form a layer, it would be an average thickness of only 3 millimeters. That is two pennies deep. Scientists say Earth’s normal ozone concentration is 300 DU for that thickness. That is represented by the large green areas on the global image above. The Ozone Hole in blue and violet has less ozone concentration in the atmosphere. The value is down to about 100 DU. That is a third of what is found over the rest of the globe.
During the Antarctic winter, the ozone concentration there is about the same as everywhere else on Earth, 300 DU. As the Sun shines more and more during the Antarctic summer, the concentration decreases from the interactions between ultraviolet-B rays and the molecules. By September, the value has decreased to about 100 DU. This low value is referred to as a hole in the concentration map.
Here is a video of the 2013 year values. Watch the change from green to blue. Watch the small charts of concentration and area. Replay as needed. It only lasts 8 seconds. Switch to HD with the gear button at lower right of the video. Every year this cycle occurs.
Historical Changes in Ozone Since 1979
Some history of the study of ozone can be found in this link.
In 1973, scientists suspected a man-made link to the decrease of ozone in the atmosphere. It was believed that chlorofluorocarbons (CFCs) were accumulating in the atmosphere, interacting with sunlight, and releasing large quantities of chlorine. The chlorine reacted with the ozone to decrease it in turn. Hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) were also found to be responsible. As a result of this research, an international treaty was opened for signature on 16 September 1987 called the Montreal Protocol banning several ozone destroying substances.
It has been a long and slow process of recovery for Earth. Here are snapshots of the Ozone Hole from four different years. The size of the hole has increased. The concentration in Dobson Units has decreased. But, their changes have slowed. If you want to see a year to year animation of the hole from 1979 to 2013, follow this link.

I obtained a table of values from NASA of the areal extend of the hole as well as the concentration in Dobson Units for September 1979 – 2013. These were charted below. A trend curve was added to each plot. Both trends have reversed and show some improvement. The ozone concentration each year is slowly recovering. The areal extend of the hole is slightly smaller in recent years. Neither of these improvements is large. But, the trend is present. We need to continue to adhere to the Montreal Protocol and encourage all nations to do their best to restrict the use of these harmful chemicals in their growing populations. We have a long way to go.


Thanks. What can we do as consumers to help?
Encourage elected officials to stay committed to the Montreal Protocols. Most of these chemicals don’t occur naturally. Fortunately, most of the major sources have been identified and prohibited. Quoting from the Clean Air Strategic Alliance in Alberta several years ago…
“Substitutes already have been found for certain uses of chlorofluorocarbons. In 1980 Canada banned their use as propellants in consumer items such as hair sprays, deodorants and antiperspirants. Major producers of polystyrene insulation were to switch to a chlorofluorocarbon substitute by the end of 1989 and the world’s largest manufacturer of chlorofluorocarbons is to phase out their manufacture by the year 2000. A major automobile manufacturer has announced that it will equip its auto dealers with chlorofluorocarbon recovery and recycling systems for servicing vehicle air conditioners. Market responses are proceeding so quickly that the reductions proposed by The Montreal Protocol will be exceeded.”
http://dwb.unl.edu/teacher/nsf/c09/c09links/www.casahome.org/chlorofl.htm
I’ll always associate ozone with the smell I remember from the electric trains I played with as a child so long ago. And now, with scent replaced by taste (which depends heavily on the sense of smell), I’m reminded of the episode of Proust’s madeleine, a kind of small cake. Here’s what Wikipedia says:
In In Search of Lost Time (also known as Remembrance of Things Past), author Marcel Proust uses madeleines to contrast involuntary memory with voluntary memory. The latter designates memories retrieved by “intelligence,” that is, memories produced by putting conscious effort into remembering events, people, and places. Proust’s narrator laments that such memories are inevitably partial, and do not bear the “essence” of the past. The most famous instance of involuntary memory by Proust is known as the “episode of the madeleine,” yet there are at least half a dozen other examples in In Search of Lost Time.
No sooner had the warm liquid mixed with the crumbs touched my palate than a shudder ran through me and I stopped, intent upon the extraordinary thing that was happening to me. An exquisite pleasure had invaded my senses, something isolated, detached, with no suggestion of its origin. And at once the vicissitudes of life had become indifferent to me, its disasters innocuous, its brevity illusory – this new sensation having had on me the effect which love has of filling me with a precious essence; or rather this essence was not in me it was me. … Whence did it come? What did it mean? How could I seize and apprehend it? … And suddenly the memory revealed itself. The taste was that of the little piece of madeleine which on Sunday mornings at Combray (because on those mornings I did not go out before mass), when I went to say good morning to her in her bedroom, my aunt Léonie used to give me, dipping it first in her own cup of tea or tisane. The sight of the little madeleine had recalled nothing to my mind before I tasted it. And all from my cup of tea.
—Marcel Proust, In Search of Lost Time
And all this came to mind because you mentioned the smell of ozone.
Interesting, isn’t it? Our sense of smell is very strongly linked to memories. I also have those about electric trains. My 38 yrs of science teaching included some spark generators to zap things and the kids. One made sparks 6″ long on a good dry day. The smell permeated the room around the demo table. Kind of intoxicating in a way. I have visions of Gene Wilder in Young Frankenstein at this time. 🙂
I live in the land of ozone alerts. Together with smoke from agricultural or prairie burns, they’re the most irritating aspect of my work – in every sense of that word. Burning eyes, cough, an irritated throat all make it extremely unpleasant to work outdoors during times of heavy concentration.
There has been progress, of course. When I first moved to Houston in 1973, there was a little saying about the area in the heart of the petro-chemical industry here. “The air is always greener in downtown Pasadener [Pasadena]”, we said. And it was. People who didn’t know much about meteorology knew the word “inversion”, and if they heard the word, they stayed indoors.
Things have improved so much over the past forty years. I hate “ozone days”, but I rarely get driven indoors any more. I checked and found that Houston had 35 ozone alerts in 2012. In Austin, where Steve lives, there were only seven. We pay a price for our industries, but at least things are improving.
I’m glad to hear your first-hand report of improvement. I would not like being surrounded by those industries. I remember visiting my brother in Los Angeles in 1963 when I was a teen. My eyes burned most days. Very unpleasant stuff.
Thank you for those comments. Let’s keep improving.
Informative post … When was the first ozone reading taken?
That is hard to say. Here is a paper of some history of ozone. http://acdb-ext.gsfc.nasa.gov/People/Stolarski/history.html
It was observed in conjunction with lightning and sparks for a long time. By the 1870s, some tests for it were known. By the 1920s, a new type of spectrometer allowed atmospheric measurements. The International Geophysical Year (IGY) in 1957 was a big step in monitoring
By the 1970s, stratospheric measurements were more routinely being made. This was still before the CFC issue came to the forefront.
No doubt, this is more answer than you wanted. But…thanks for asking.
I had no clue about any sort of guideline, so your stab at it is a start. Thanks!
Thanks for this article, I needed a review on this.